Pharmaceutical Monitoring: At-Line, Quantitative, Multiplexed Data
Customized and Multiplexed Tracking of Proteins, Small Molecules, and other Process Parameters.
We use VHHs in an automated immunoassay to quantify proteins and other targets at low concentrations, autonomously and at-line.
Our sensors have been real-world tested in many locations including the EPA’s Test and Evaluation Facility in Cincinnati (right).
Our sensor automates a VHH-based agglutination assay that can be used at-line to track concentrations of any analyte an antibody can bind.
Our platform has been tested in many real-world environments.
Pictures at right show integration with an algae pond to represent dirty, turbid water and the recirculation loop at the EPA’s Test and Evaluation Facility in Cincinnati, OH.
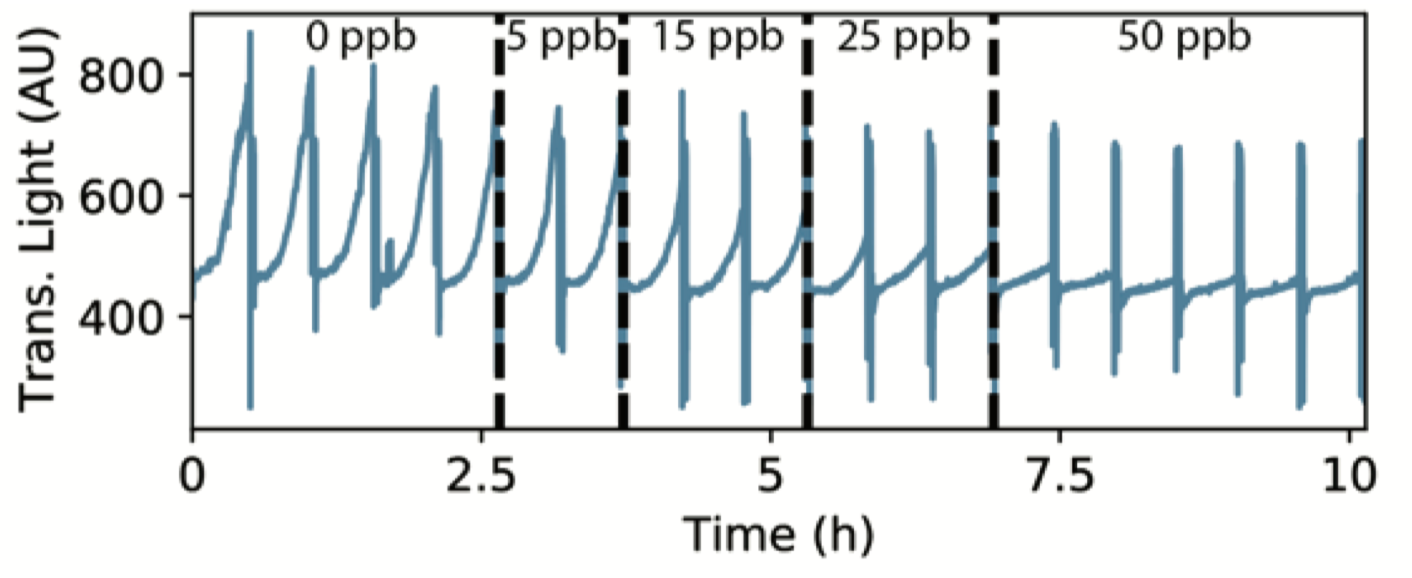
Proof of principle was demonstrated with at-line drug-of-abuse and caffeine monitoring at two different sites
Target was quantified every 15 minutes using VHH-mediated agglutination
Data can be accessed and monitored remotely
Platform can reduce need for process disruption and can eliminate delays, providing actionable data automatically
Potential Applications in Process Monitoring:
Increase throughput: monitor and “smart-dose” analytes automatically, reducing labor and increasing productivity
Quality control: monitor protein production in real time, detect contaminants, or even detect improper folding
Early warning: save batches via early detection of problematic contaminants and/or imbalanced process parameters
Monitor water treatment or wastewater for pharmaceuticals or other contaminants